- orthopaedic surgery
Autologous Cultured Cartilage Transplantation for Joint Repair【Kameda Medical Center】

Dr. Kato
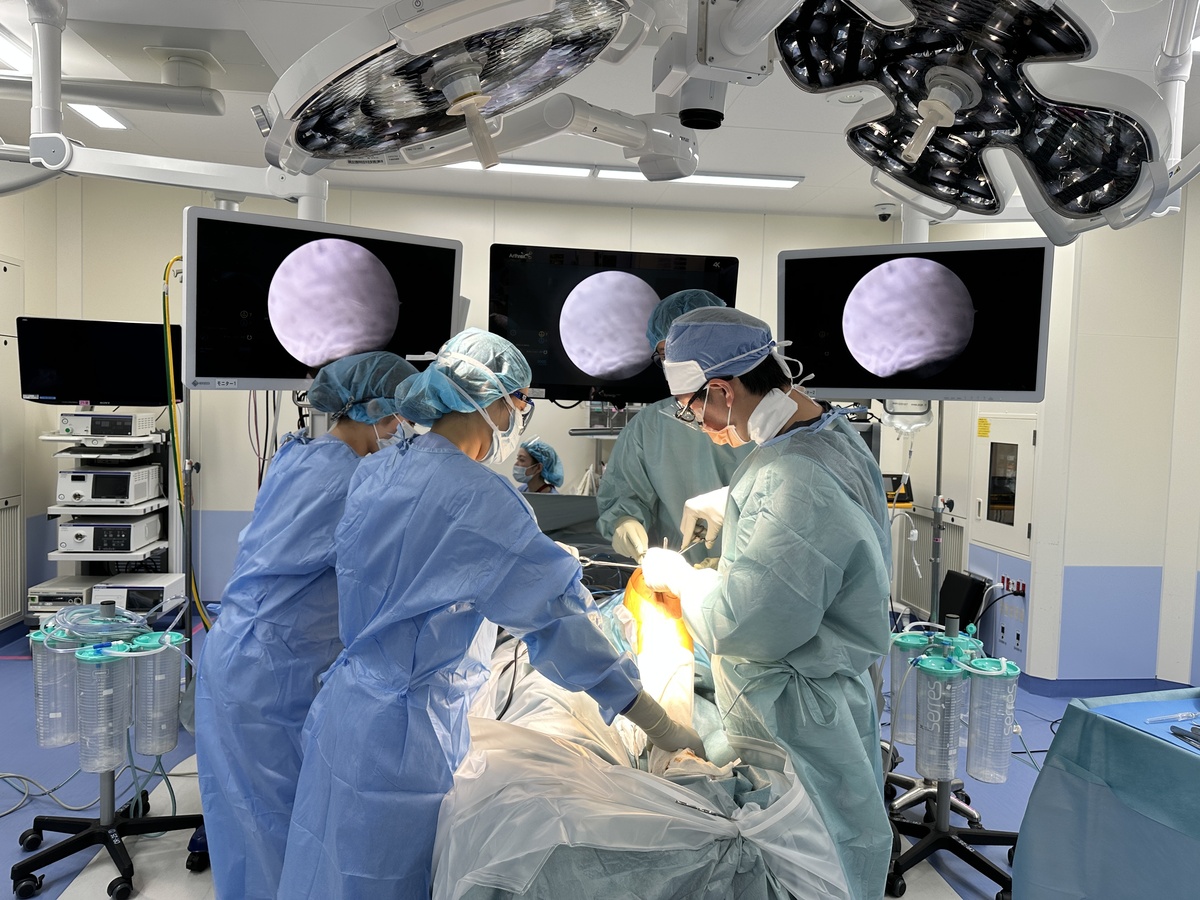
Surgery

Exterior view of Kameda General Hospital

Dr. Kato

Surgery

Exterior view of Kameda General Hospital
for more information about this program,
please contact us here
please contact us here
other program on this facility

