JTB Medical & Healthcare supports the development of this world’s first service providing from Japan.
We recommend you to generate and store iPS cell for future medical treatment.

iPS cells are expected to be used in a variety of treatment, and medical treatments or clinical trials (administration to humans) using iPS cells have started in Japan, Europe, and the United States. A future where diseases that were once untreatable can be cured by regenerative medicine using iPS cells is becoming a reality. It is important for the treatment of disease and injury to provide appropriate treatment promptly at the necessary time. However, it is reported that iPS cells used in regenerative medicine take time to be produced and along with cell’s aging, production efficiency decreases. Storing personal iPS cells in advance can shorten the preparation time by the start of the treatment, which means treatment can be started quickly. We recommend you to prepare it for the future.
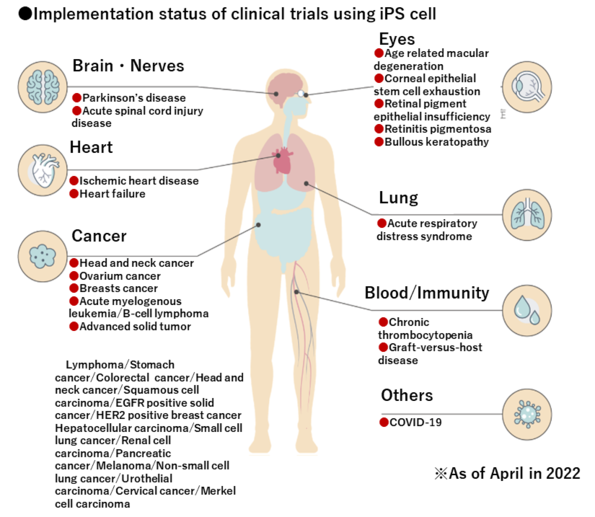
iPS cells that the development to various regenerative medicine is advancing
Since Prof. Shinya Yamanaka invented human iPS cells, researches and clinical trials using iPS cells have performed all over the world. Recently, a lot of practical research and development has been carried out, including the application of iPS cells to regenerative medicine. In Japan, clinical trials and clinical researches (administration to humans) using iPS cells for age-related macular degeneration, Parkinson’s disease, and heart disease have already started. The development of treatment methods using iPS cells is progressing day by day, and there are many that are at the stage of administration to humans. A future where regenerative medicine using iPS cells can cure many diseases that were once thought to be incurable is becoming a reality.
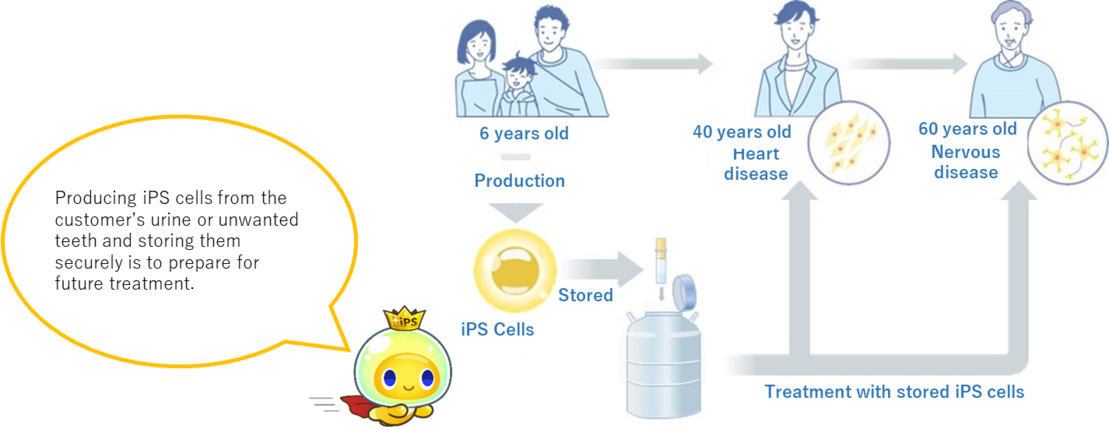
How personal iPS work
iPS cell made from unwanted teeth or urine
Since it is possible to produce iPS cells from cells (dental pulp cells) contained in the inside of unwanted teeth or cells (UPC) contained urine, there is no burden on the body, and so anyone even children can produce iPS cells easily. Inside of teeth or urine is fundamentally sterile and contains dental pulp cells and UPC which are the source of iPS cells. After collecting these cells, we will culture the cells until we reach them to a certain amount to produce iPS cells. Then, the cultured cells are given reprogramming* factors necessary for iPS cell formation. And we will create iPS cells. Reprogramming*=Changing somatic cells into iPS cells
Avoiding the risk of rejection
Transplantation (autologous transplantation) treatment using the patient’s own cells is less likely to cause rejection. As Personal iPS creates iPS cells from your own cells, rejection does not occur. In addition, there is no need to search for donors during transplant surgery, and treatment can be proceeded quickly. Taking immunosuppressive drugs is not necessary, which improves patient’s QOL, and it is also an advantage.
Reducing time to treatment
In general, it is said that it takes several months to half a year to get iPS cells and ensure their quality. Some injuries and diseases, such as spinal cord injury or central nervous system disease, has a better prognosis if treated as early as possible. By creating and storing cells with Personal iPS, it is possible to make thorough preparation in case of injury or illness, and start treatment early. Creating and storing iPS cells from your own healthy cells while you are young is able to make you prepare for treatment of various injuries and illnesses. And even if you do get injured or become ill, you can rest assured that you will be able to get treatment.
The younger you are, the fewer genetic mutations you have.
In general, it is said that the older you get, the more mutations occur in your genes, causing various disorders. The most representative of these is “cancer”. When looking at the cancer incidence rate by age group, there are statistics that the cancer incidence rate increases with age. In fact, when iPS cells were produced from people of various age, it was reported that the older the cell donor, the greater the number of gene mutations.
Personal iPS service cost details
The cost of producing personal iPS and the cost of storing iPS cells for 10 years and quality control are required.
A plan “Personal iPS Dental” made from teeth and a plan “Personal iPS urine” made from urine are offerd. Please submit your teeth or urine. The timing of submission will be confirmed and determined at the time of application.
The contract term for initial cell storage is 10 years, however, storage can be continued after that. In addition, there are many typhoons and earthquakes in Japan so that it can not be denied that stored iPS cells may be exposed to danger. For this reason, we cultivate a large amount of the iPS cells we have created and store them in two bases in Japan and the US just in case, to ensure the protection of our customer’s iPS cells.
Service procedures (for foreign visitors to Japan)
Those who do not have a Japanese health insurance card (those who do not have a Japanese resident card) Those who do not read and write in Japanese for communication or understanding contents of manual・consent form)
Please check the instruction manual for this product before applying.
Please confirm the contents of the contract and consent form, agree in advance, and pay the production and storage management costs.
You may be asked to pay the inspection fee in advance. Please confirm when applying.
You may submit your examination results for infectious disease before coming to Japan. If you wish, please contact us regarding the examination items.
Urine collection・infectious disease examination point
After「 ② confirmation of contract details and payment of fees」, we will arrange the location for urine collection and infectious disease testing. (In case of manufacturing using teeth, there are prior conditions.)
In terms of provision of regenerative medical information after cell storage
We are planning to send out information regarding iPS cell regenerative medicine to the email address registered when the contract is concluded. When using it for treatment, please contact our center after consulting with your doctor in charge.
Use for regenerative medicine
iPS cells produced and stored by Personal iPS can be expected to be used for regenerative medicine treatment by policyholders themselves in the future. Treatment is necessary for the policyholder, and if the regenerative medicine information after cell storage indicates a target disease, please consult with your doctor and contact our center. We will coordinate with the medical institution that is the target of the treatment and whether or not iPS cell therapy can be applied (a separate condition fee will be incurred).
Contact information for this service:JTB Japan Medical & Health tourism Center (JMHC)
Personal iPS is a registered trade mark owned by Riprocell I Co., Ltd., and is a service created by consolidating cutting-edge technology not only from Japan but also from around the world. Since its founding in 2003, Reprocell Co., Ltd. has been engaged in research and development related to ES cells and iPS cells for many years, and its cell culture fluid has been used in the research of Nobel Prize-winning Professoe Yamanaka. To date, Reprocell’s products have been cited in more than 6000 papers (as of November 2020). The contents of Personal iPS on this site are posted with permission based on information from Reprocell Inc. under a contract with our company.
Please refrain from reprinting or diversion without permission.Please contact our center for details.